Design Methodology
Composite Alginate-Gelatin Bioink
The basis of this design incorporates two different bioink formulations – a stiff bioink and a more fluid bioink. The stiff bioink should provide better structural properties to the construct. A lower viscosity bioink should allow the cells to survive the printing process since the pressure needed to print the stiff ink would result in cell death.
This idea is based off another study by Jodat et. al. that used two bioinks of differing stiffnesses composed of GelMa, PEGDMA, and gelatin [8]. This project will create two bioinks with both bioinks will be composed of alginate and gelatin at different concentrations. Alginate is a low cost, biocompatible, non-toxic, natural polymer hydrogel [9]. Alginate can be crosslinked using calcium chloride (CaCl2), which is also biocompatible. One study looked at the concentrations on CaCl2 and how it affects the crosslinking of alginate. They saw that 0.8M CaCl2 led to an improvement in the mechanical properties of sodium alginate films [10]. Gelatin is another biocompatible compound; however, it is liquid at temperatures above approximately 32°C [11]. This means that to culture the constructs with cells (which must be kept at physiological temperature of ~37°C) the gelatin must also be crosslinked. This can be done using genipin, which has been shown to be able to crosslink gelatin films at a concentration of approximately 0.67% [12].
The stiff bioink will have 5% alginate and 10% gelatin (5A10G) (w/v), the more fluid bioink will have 3% alginate and 5% gelatin (3A5G) (w/v). Both bioinks are made using distilled water. These formulations were chosen based off a study conducted by Liu et.al. that looked at the stiffness of alginate-gelatin bioinks. Liu et. al. found that 5A10G had a Young’s modulus of 355kPa, and 3A5G had a Young’s modulus of 165kPa [13]. This is lower, although comparable to the Young’s modulus of native cartilage, which is within the range of 450-800 kPa [14]. The crosslinker is comprised of 5% w/v CaCl2 and 0.7% w/v genipin, mixed with TBS.
| Material | Component | Concentration (w/v) |
|---|---|---|
| Material | Component | Concentration (w/v) |
| Bioink (stiff) | Alginate Gelatin | 5% 10% |
| Bioink (fluid) | Alginate Gelatin | 3% 5% |
| Crosslinker | CaCl2 Genipin | 5% 0.7% |
The process for making 10ml of the 3A5G (5A10G) bioink is as follows:
- Weigh out 300mg (500mg) of alginate and 500mg (1000mg) of gelatin
- Add 10mL distilled water
- Set the hot/stir plate to 115°C and 600RPM. Add gelatin to beaker and stir untl dissolved ~ 15-20 mins. Cover to avoid evaporation.
- Add the alginate. Add the alginate very slowly to avoid clumping.
- Cover the mixture and allow the mixture to stir at 600RPM and 115°C for 60-90mins, until a homogenous mixture is reached.
- Once the components are fully incorporated, remove the bioink from the hot/stir plate. The bioink must cool before use.
The cost for making 10mL of both 3A5G and 5A10G bioinks is outlined below
| Component | Cost per container ($) | Cost per gram ($) | Cost per mL of bioink ($) |
| Alginic Acid Sodium Salt (100g container) | 69.3 [15] | 0.693 | 0.5544 |
| Gelatin from porcine (100g container) | 81.60 [16] | 0.816 | 1.224 |
| Total Cost | 147.6 | 1.476 | 1.7784 |
Analysis and Testing Validation
Live Dead Assays
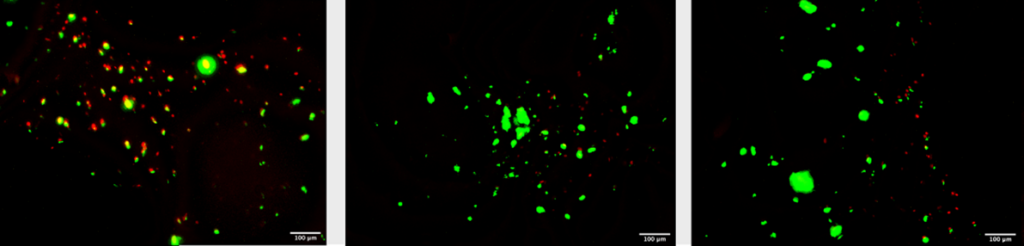
Fluorescence microscopy was done on the constructs to determine cell viability. Live/dead staining was done using a green/red staining and fluorescence microscopy. The green stain was Calcein AM which indicates intracellular esterase activity showing a live cell. The red stain was EthD-III which indicates a loss of plasma membrane integrity showing a dead cell. Using this method cell viability was determined to be approximately 55% on day 1. This method was complicated by gelatins autofluorescence at the red dyes excitation wavelength of 494nm. It’s found that spindle-liked morphology was dominant. The stiffness of bioink did affect the cultivation of hAC in the absence of TGF-ß3 [17]
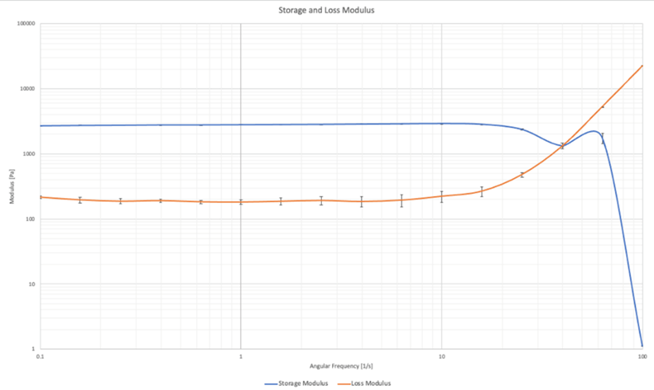
Rheological Analysis of the Scaffold
Rheological testing was done on five constructs. The loss modulus, storage modulus and viscoelasticity were able to be directly measured, the elastic properties were then able to be calculated from the results. Of the five constructs only two gave meaningful data. The infill of the print was set to 25% which can result in inconsistent data when undergoing destructive testing.
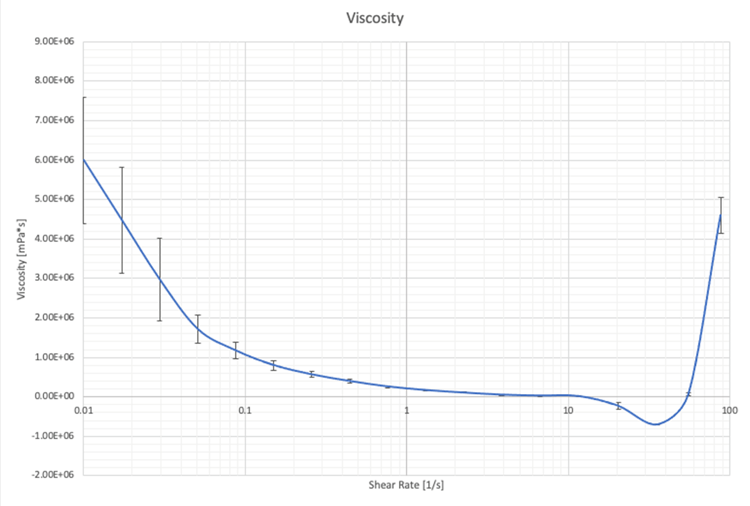
Viscoelasticity
Shear viscosity can also be measured using oscillatory rheology. It is the ability of a material to resist shear stress. Shear thinning is when the fluid viscosity decreases with increasing shear. From Figure 3 this can be seen at low shear rates. However, from Figure 3 the critical shear where shear thinning begins cannot be seen.
Cost Analysis
The direct costs, indirect costs, and funding are shown below in Table 1. The highest cost came from the purchasing of MSCs and labour. The costs of MSCs is set and can not be lowered however labour costs can be lowered with more experience and by doing more prep work outside of the laboratory.
Cost table (direct costs = DC; indirect costs = IC; funding = F)
| Sources | $ [Unit] | Type |
| MSCs come with medium | 126?? | DC |
| Alginic acid Sodium Salt | 69.3 [100g] | F by laboratory |
| Gelatin from porcine | 81.6 [100g] | F by laboratory |
| BIO-X rent | 290 [1 day] | F by laboratory |
| Cell culturing stereo environment | ?? | F by laboratory |
| Working hours | 720 [45 hr] | ID |
| Material used for passaging | 80 | ID |
| Other (flasks, vials, pipettes, etc.) | 50 | ID |
The cost to develop this project to market could be over $100 million [18]. This cost would include initial engineering development, in vitro and in vivo testing and FDA approval. This is a very large and daunting number but the US CDC estimates that the cost of osteoarthritis to be $303.5 billion [19]. If this project is successfully brought to market the potential return on investment is great as well as enormous quality of life improvement for people living with osteoarthritis.
Recent Comments